February Bulletin
Issue 101
Community Notices
Next Marble Center seminar on February 3rd
Next Marble Center seminar is on Monday February 3 (4-5pm) at the KI Luria Auditorium with a research talk by Justin Kaskow of the Hammond lab on "Nanoscale Polymer Complexes Deliver STING Protein Fragments to Activate Innate Immunity in Ovarian Cancer.”
Following the talk, we will have a hot topic presentation by Dr. Maggie Billingsley (Hammond Lab) on the promise and pitfalls of multi-cancer early detection (MCED) tests.
News
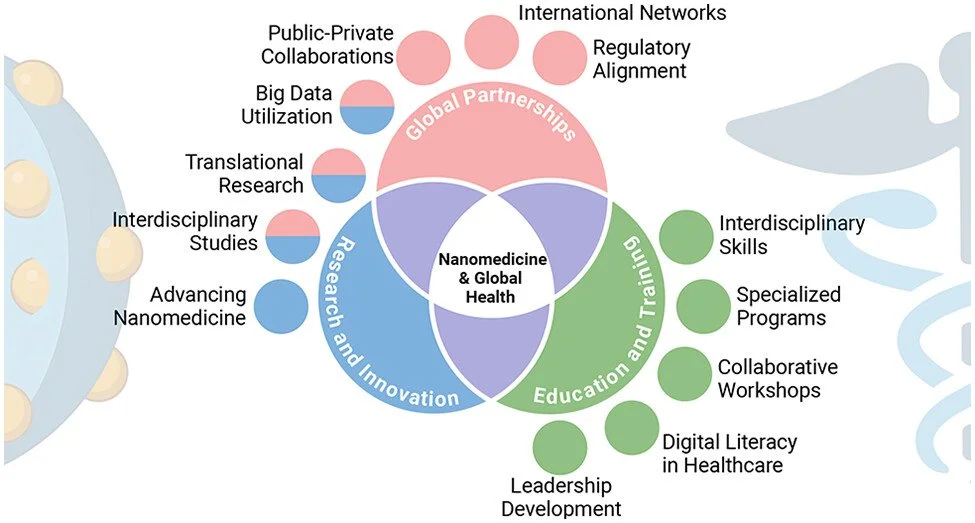
Voices of Nanomedicine: Blueprint Guidelines for Collaboration in Addressing Global Unmet Medical Needs
(Adapted from abstract) The “Voices” under this Perspective underline the importance of interdisciplinary collaboration and partnerships across several disciplines, such as medical science and technology, medicine, bioengineering, and computational approaches, in bridging the gap between research, manufacturing, and clinical applications. Effective communication is key to bridging team gaps, enhancing trust, and resolving conflicts, thereby fostering teamwork and individual growth toward shared goals. Drawing from the success of the COVID-19 vaccine development, the authors advocate for the application of similar collaborative models in other complex health areas such as nanomedicine and biomedical engineering. The role of digital technology and big data in healthcare innovation is highlighted along with the necessity for specialized education in collaborative practices. This approach is decisive in advancing healthcare solutions, leading to improved treatment and patient outcomes.
Bob Langer awarded the 2025 Lipid Science Prize from the Camurus Lipid Research Foundation
(June 27, 2025) Camurus Lipid Research Foundation has awarded the 2025 Lipid Prize to Professor Robert S. Langer (MIT). Dr. Langer receives the award for his groundbreaking and interdisciplinary research focused on the design and characterization of complex materials, including lipid and polymer systems, and the application of these in drug-delivery, tissue engineering and regenerative medicine. The work of Dr. Langer has resulted in more than 1500 publications, numerous patents, and the founding of over 40 biotechnology companies. In addition, he has mentored many students, postdocs, and researchers, who are now established leaders in academia and industry. Read more…
Estée Lauder Companies partners with the Langer Lab to drive ingredient innovation across suncare and biodegradable product formulations
(Lexy Lebsack, Glossy) The Estée Lauder Companies is partnering with Massachusetts Institute of Technology’s renowned Langer Lab to fuel ingredient innovation.
The new long-term partnership will center around the advancement of biodegradable polymers primed to replace common personal-care ingredients found in cleansers, sun-care, cosmetics and more. The partnership will also focus on studying new solutions to help combat the effects of visible sunlight and blue light on skin.
“MIT is one of the world’s leading institutions in many fields of science, and in particular, in the invention of materials,” Carl Haney, ELC’s evp of research, product and innovation, told Glossy. MIT’s Langer Lab is overseen by Robert S. Langer, a researcher with over 1,000 patents who is often credited as the most cited engineer in history, according to the Science History Institute. Dr. Langer’s patents have been licensed or sublicensed to over 400 pharmaceutical, chemical, biotechnology and medical device companies, according to MIT. Read more…
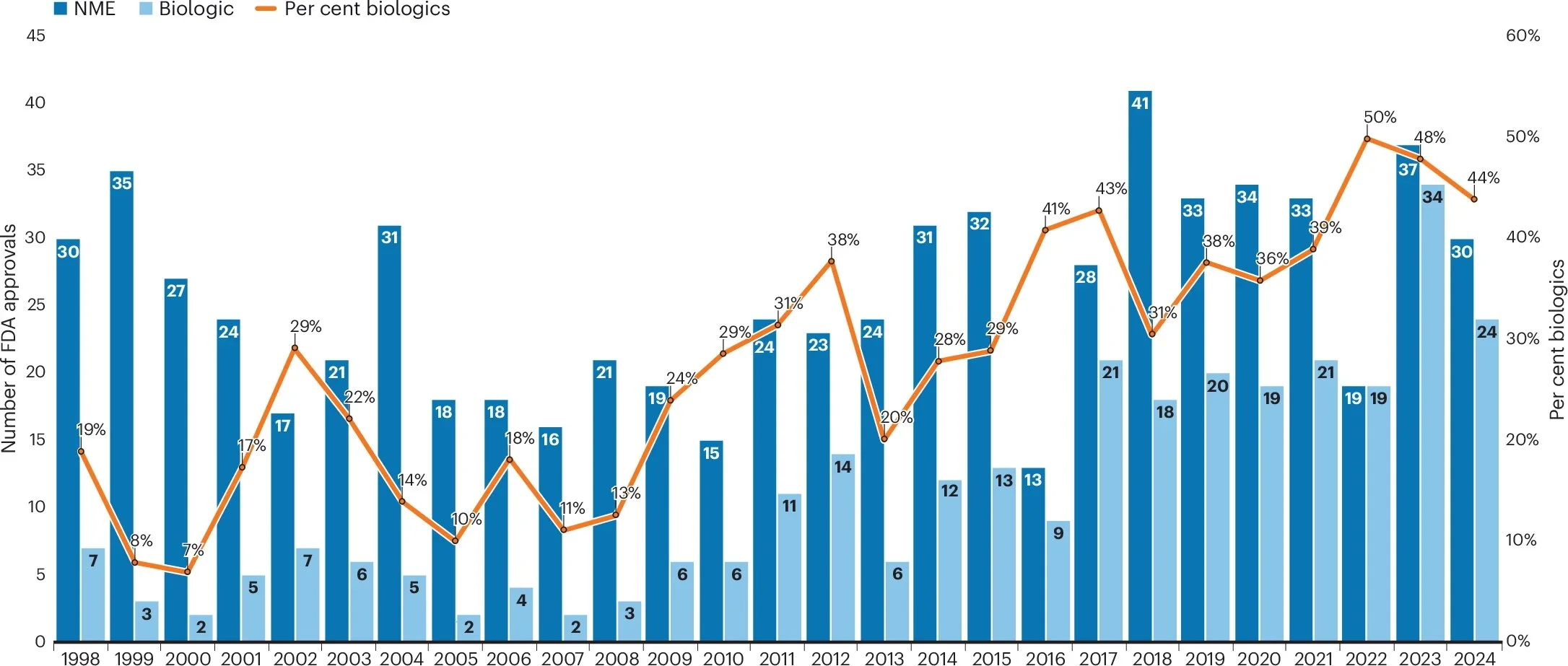
FDA approvals settle in 2024, but what’s next?
(Melanie Senior, Nature Biotechnology) Almost two-thirds of the 54 new therapies and vaccines indeed emerged from biotech, rather than Big Pharma, pipelines, marking a shift from 2023 and highlighting biotechs’ commercial ambitions. Rare disease drugs made up over half of all new medicines, as has become typical. But there were notable new solutions for widespread chronic conditions, including metabolic dysfunction–associated steatohepatitis (MASH, formerly known as nonalcoholic steatohepatitis (NASH)), schizophrenia, Alzheimer’s disease, chronic obstructive pulmonary disease (COPD) and hypertension. Four anti-infectives were approved. Sufferers of moderate to severe eczema got a new injectable treatment, and a third option arrived for the estimated 6.7 million Americans with autoimmune-linked hair loss.
FDA new drug approvals (CDER and CBER): The FDA approved fewer drugs in 2024 than in 2023.
2024’s tally included fewer next-generation modalities than in 2023, but the year nevertheless brought trios of new gene therapies, cell therapies and bispecific antibodies, a pair of fusion proteins and two oligonucleotide-based drugs, highlighting an increasingly multimodal medicine cabinet. There were no new antibody–drug conjugates (ADCs) or radioligand therapies in 2024; these remain, for now, more prominent on dealmakers’ tables than in regulators’ in-trays. Biologics’ share of approvals, which had reached an all-time high of 50% in 2022, dipped to 44% in 2024. Almost two-thirds (65%) of the Center for Drug Evaluation and Research (CDER)’s 46 novel drug approvals were for small molecules submitted via New Drug Applications (NDAs) — a reminder that orally administered therapies remain attractive, despite their asymmetric treatment by the US Inflation Reduction Act, which grants them a shorter exclusivity period than biologics prior to Medicare price negotiations. Read more…
2024’s new drugs for big diseases
Multimedia
January hot topic: Next-generation vaccines for infectious diseases
Job opportunities
Associate Director, Office of Strategic Partnerships. The Associate Director will be responsible for planning and management of activities of the Strategic Partnerships Office, including supporting and overseeing strategic initiatives at the Institute level, and identifying and prioritizing new cross-cutting strategic initiatives. This role will require collaboration and coordination across multiple teams at the Institute and will involve establishing partnerships and alliances with external parties including academic centers and industry. This position reports directly to the Chief of Strategic Partnerships. This is an exciting opportunity to help drive strategic initiatives that support our Institute’s mission to improve the lives of cancer patients. Under supervision of the Office of Strategic Partnerships, the Associate Director will help develop and coordinate efforts around strategic initiatives. This role will work collaboratively with the Associate Director of the Center for Cancer Therapeutic Innovation (CCTI) as well as with other associate directors, directors, faculty and staff within DFCI. This role may involve setting goals and timelines for initiatives, delineating resource and budget needs, tracking progress, as well as writing and editing related to internal and external communications.
Postdoctoral Associate, Alnylam Pharmaceuticals. The Research Department at Alnylam is seeking a self-motivated, innovative postdoctoral fellow to advancing fundamental understanding of mechanisms in the context of applications beyond gene silencing. This role also requires supporting developing cutting-edge technologies in the oligonucleotide field, aimed at creating innovative therapeutics for unmet medical needs. The successful candidate will have a strong experimental background in cell/molecular biology and biochemistry, with a focus on small RNA biology or gene editing related technologies, and brings a rigorous, analytical approach to problem solving and experimental design. It is expected that the research conducted by the postdoctoral fellow will result in high impact publications after filing appropriate patents and contributing to the Alnylam pipeline. The ideal candidate will possess exceptional collaboration and communication skills, effectively bridging team members across various disciplines and working closely with internal/external partners. A strong foundation in molecular biology/biochemistry, and gene editing technologies such as RNA editing/CRISPR/DNA base editing is essential.
Funding opportunities
| Funding Source | The Mark Foundation Early Cancer Detection Award (LOI) | March 10, 2025 | American Cancer Society Postdoctoral Fellowship | April 1, 2025 |
|---|